Retinaldehyde
Introduction
Retinoic acid, a derivative of vitamin A, is a crucial molecule that plays a fundamental role in various biological processes. This compound is involved in cell differentiation, growth, and development, making it essential for the proper functioning of living organisms. In this essay, we will delve into the significance of retinoic acid in biology, focusing on its different forms and functions. Specifically, we will explore the roles of all-trans and 9-cis retinoic acid, two distinct forms with specific functions in cellular processes. By understanding the biological functions of retinoic acid and analyzing its chemical structure, we aim to gain a deeper insight into how this molecule influences and regulates key biological pathways. Through this exploration, we will uncover the intricate mechanisms by which retinoic acid contributes to the development and maintenance of living organisms. Join us on this journey as we unravel the complexities of retinoic acid and its vital role in biological processes.
The Function of Retinoic Acid
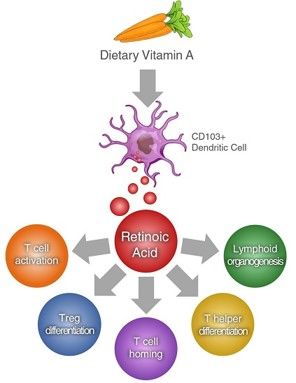
Retinoic acid plays a crucial role in cell differentiation by activating nuclear receptors and inducing the transcription of relevant genes. This process is integral to the growth and development of various organs and systems in the body. As highlighted in the study by Zasada and Budzisz (2019), retinoids, including retinoic acid, are actively involved in embryogenesis, particularly in the development of crucial organs such as the nervous system, liver, heart, kidneys, and eyes. Moreover, retinoic acid is essential in ensuring proper functioning of the organ of sight, making it a key player in maintaining biological functions. Additionally, retinoic acid's interaction with keratinocytes, fibroblasts, melanocytes, and Langerhans cells enhances cellular activity, promotes proliferation, and strengthens the epidermal protective function, showcasing its multifaceted impact on various biological processes within the body.
Here are some key points about retinoic acid:
Diverse Forms of Retinoic Acid
All-trans retinoic acid (RA) and 9-cis retinoic acid are crucial components in various biological processes. RA, the physiologically active form of retinoids, serves multiple functions in vision, embryonic development, adult growth, and reproduction in both males and females. It acts as a nutrient-derived vitamin, but also functions as a hormone through retinoid-specific nuclear receptors, influencing gene transcription. On the other hand, 9-cis retinoic acid plays a distinct role in maintaining tissue levels of RA through regulated synthesis, catabolism, and proper uptake mechanisms. According to Nilsson and Jacobs, RA interacts primarily with nuclear retinoid acid receptors (RARs) and forms heterodimers with retinoid X receptors (RXR), which are crucial for binding to specific DNA response elements. The extensive cross-talk between nuclear receptor pathways, facilitated by RXR, underscores the significance of understanding the different forms of retinoic acid for advancing research and medical applications in the field (7).
Chemical Structure of Retinoic Acid
Retinoic acid, with the molecular formula C20H28O2, contains a cyclohexenyl ring with an attached hydroxyl group and a carboxylic acid functional group. These key structural features play a crucial role in the biological activity of retinoic acid. The cyclohexenyl ring allows for the molecule's binding to specific nuclear receptors, such as Retinoid X Receptors (RXR) and Retinoic Acid Receptors (RAR), influencing gene transcription and cellular responses. Furthermore, the hydroxyl and carboxylic acid groups enable retinoic acid to interact with cellular proteins, leading to downstream effects on cell differentiation and proliferation. As highlighted by Zasada and Budzisz (2019), retinoic acid's structural characteristics are essential for its ability to modulate gene expression and impact skin structure formation in cosmetic and dermatological treatments.
Clinical Uses
Dermatology: All-trans-retinoic acid (tretinoin) is widely used in topical formulations to treat acne, psoriasis, and other skin conditions due to its ability to promote cell turnover and prevent the formation of comedones (clogged hair follicles).
Cancer Therapy: Tretinoin is also used in the treatment of acute promyelocytic leukemia (APL), a subtype of acute myeloid leukemia, where it induces the differentiation of the abnormal promyelocytes.
Isotretinoin: Used primarily for severe acne that does not respond to other treatments. It reduces sebum production, inhibits sebaceous gland activity, and has anti-inflammatory properties.
Side Effects and Considerations
Teratogenicity: Retinoic acid is highly teratogenic, meaning it can cause birth defects if taken during pregnancy. This risk necessitates careful monitoring and contraceptive measures for women of childbearing age taking retinoic acid derivatives.
Other Side Effects: Can include dry skin, photosensitivity, and mucous membrane irritation. Systemic use can lead to more severe side effects such as liver damage and elevated lipid levels.
Metabolism
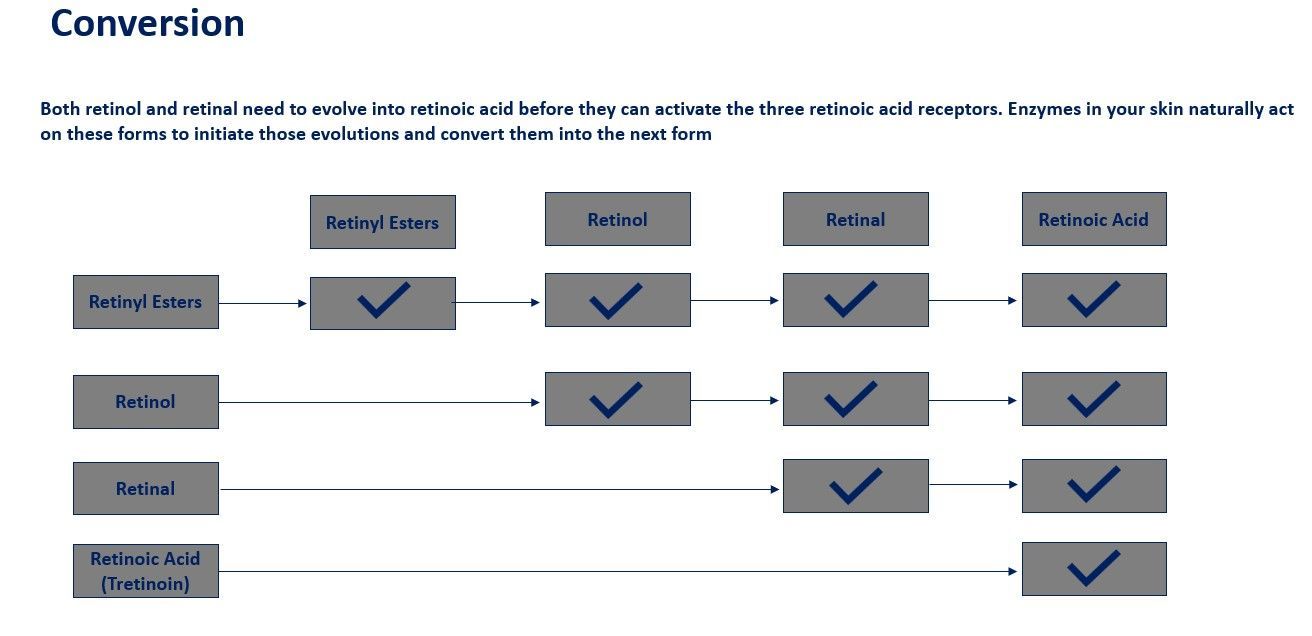
Synthesis: Retinoic acid is synthesized in the body from retinaldehyde, which in turn is derived from retinol.
Catabolism: It is metabolized in the liver and other tissues through oxidation and conjugation processes, which render it water-soluble for excretion.
Retinoic acid's ability to influence gene expression makes it a powerful molecule with significant therapeutic potential, but also with risks that require careful management in clinical settings.
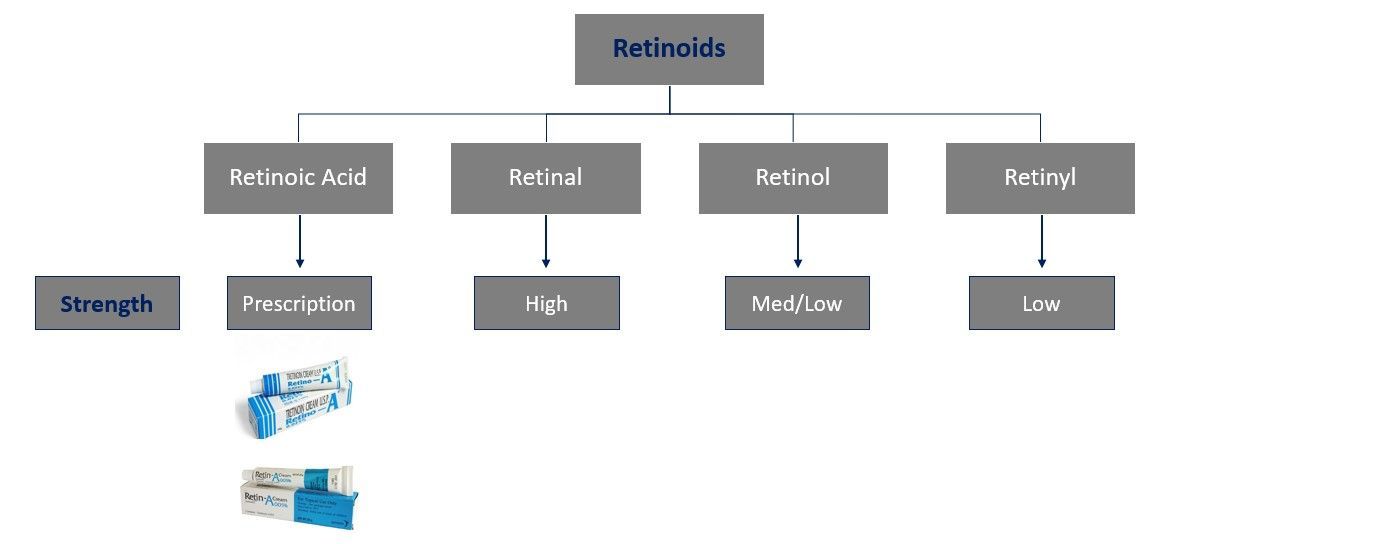
Vitamin A exists in several forms, each with distinct structures and functions. These forms can be broadly categorized into two groups: preformed vitamin A (retinoids) and provitamin A (carotenoids).
Preformed Vitamin A (Retinoids)
These are the active forms of vitamin A that can be directly utilized by the body. They are found in animal products and include:
Retinol
Structure: Retinol is an alcohol form of vitamin A.
Functions: It serves as a storage form of vitamin A and can be converted into retinal (for vision) or retinoic acid (for gene expression and cellular functions).
Sources: Liver, fish oils, eggs, dairy products.
Retinal (Retinaldehyde)
Structure: Retinal is an aldehyde form of vitamin A.
Functions:Crucial for vision, retinal combines with opsin proteins to form rhodopsin, a pigment necessary for low-light and color vision.
Conversion:Retinol can be oxidized to form retinal, which can then be further oxidized to retinoic acid or reduced back to retinol.
Retinoic Acid
Structure:Retinoic acid is a carboxylic acid form of vitamin A.
Functions: It does not participate in the visual cycle but is vital for gene regulation, cellular differentiation, and embryonic development.
Forms:
All-trans-retinoic acid (tretinoin): Used in dermatology and cancer treatment.
13-cis-retinoic acid (isotretinoin): Primarily used to treat severe acne.
9-cis-retinoic acid: Less commonly discussed but plays a role in activating retinoid X receptors (RXRs).
Provitamin A (Carotenoids)
These are precursors to vitamin A found in plant-based foods. The body converts them into active vitamin A. The most significant carotenoids are:
Beta-Carotene
Structure: Beta-carotene is a carotenoid with two retinol molecules joined together.
Functions: It is a potent antioxidant and can be enzymatically cleaved in the intestine to produce two molecules of retinol.
Sources: Carrots, sweet potatoes, spinach, kale.
Alpha-Carotene
Structure: Similar to beta-carotene but with a slight difference in the molecular structure.
Functions: It also converts to retinol, though less efficiently than beta-carotene.
Sources: Pumpkins, carrots, tangerines.
Beta-Cryptoxanthin
Structure: A less common carotenoid with provitamin A activity.
Functions: Converted to retinol in the body, but with lower efficiency compared to beta-carotene.
Sources: Papayas, oranges, red peppers.
Absorption and Metabolism
Absorption: Retinoids are absorbed in the small intestine via dietary fats and bile acids. Carotenoids are absorbed less efficiently and require enzymatic conversion to retinol.
Storage: Vitamin A is primarily stored in the liver as retinyl esters.
Transport:Retinol is transported in the bloodstream bound to retinol-binding protein (RBP) and transthyretin.
Conversion:Retinol can be converted to retinal by retinol dehydrogenases (RDH), and retinal can be further oxidized to retinoic acid by retinal dehydrogenases (RALDH).
Functions and Importance
Vision:Retinal is essential for the formation of rhodopsin in rod cells of the retina, which is crucial for night vision.
Immune Function:Vitamin A enhances the immune response and supports mucosal barriers.
Cell Growth and Differentiation:Retinoic acid regulates genes involved in cellular growth, differentiation, and apoptosis.
Reproduction: Vital for spermatogenesis in males and for preventing fetal abnormalities in females.
Retinoic acids, which are derivatives of vitamin A, play a critical role in cell growth, differentiation, and apoptosis (programmed cell death). Here's an overview of how retinoic acids work with cells:
Mechanism of Action
Uptake and Activation:
Retinoic acids are derived from dietary vitamin A (retinol). Retinol is converted into retinal and then into retinoic acid within the body.
The most active forms of retinoic acid are all-trans retinoic acid (ATRA) and 9-cis retinoic acid.
Binding to Retinoic Acid Receptors:
Retinoic acid exerts its effects by binding to specific nuclear receptors: retinoic acid receptors (RARs) and retinoid X receptors (RXRs).
These receptors are transcription factors that regulate gene expression. RARs bind to all-trans retinoic acid, while RXRs bind to 9-cis retinoic acid.
Formation of Heterodimers:
RARs and RXRs can form heterodimers (combinations of two different molecules) with each other, which are essential for their function.
These heterodimers bind to specific DNA sequences called retinoic acid response elements (RAREs) located in the promoter regions of target genes.
Regulation of Gene Expression:
Once bound to RAREs, the RAR/RXR heterodimers regulate the transcription of target genes. This regulation can either activate or repress the expression of these genes.
The genes regulated by retinoic acid are involved in a variety of cellular processes, including cell proliferation, differentiation, and apoptosis.
Cellular Effects
Cell Differentiation:
Retinoic acid promotes the differentiation of cells. For example, in the development of the nervous system, retinoic acid influences the differentiation of neural stem cells into neurons.
It also plays a crucial role in the differentiation of epithelial cells and the maintenance of epithelial tissues.
Cell Proliferation:
By regulating the expression of genes involved in the cell cycle, retinoic acid can either promote or inhibit cell proliferation.
This regulation is crucial in maintaining tissue homeostasis and preventing uncontrolled cell growth, which could lead to cancer.
Apoptosis:
Retinoic acid can induce apoptosis in certain cell types. This is particularly important in development, where the removal of unnecessary or damaged cells is required.
In cancer therapy, retinoic acid is used to promote the apoptosis of malignant cells, thereby inhibiting tumor growth.
Clinical Applications
Skin Disorders:
Topical retinoic acid is commonly used in the treatment of acne and psoriasis. It helps normalize the shedding of dead skin cells and reduces inflammation.
Retinoic acid also promotes the production of collagen, making it useful in anti-aging skin treatments.
Developmental Biology:
Retinoic acid is crucial in embryonic development, particularly in the development of the brain, spinal cord, heart, and limbs. It ensures that cells differentiate into the appropriate cell types needed for organogenesis.
In summary, retinoic acids exert their effects through binding to nuclear receptors, regulating gene expression, and influencing cellular processes such as differentiation, proliferation, and apoptosis. These actions are fundamental in both normal physiological processes and therapeutic interventions for various diseases.
Conclusion
The role of retinoic acid in regulation of critical biological processes is enormous. It is actively involved in cell difference and growth, where particular forms of retinoic acid like all-trans and 9-cis retinoic acid perform specific physiological duties. The deep chemical background of retinoic acid has allowed researchers to therefore begin understanding its actions on different cellular pathways and have a big effect on both normal and pathological conditions through the mastery of gene expression, development, and maintenance of tissues. The vast areas for its use have become uncovered by means of its participation in processes like embryonic growth, immune function, and the health of the skin. Research is being extensively conducted on the potential of using the retinoic acid in different medical areas from fighting certain shapes of cancers to treating skin troubles and neurodegenerative troubles. With the further understanding of retinoic acid, new uses for them in clinical and basic science keep appearing and may result in developing new ways to treat conditions and improve fields like regenerative health and personalized healthcare. The subject of examining retinoic acid still offers great potential for developing new treatments and investigating cellular pathways with the goal of improving the health and well-being of people.
Works Cited
Zasada, Malwina, and Elżbieta Budzisz. "Retinoids: Active Molecules Influencing Skin Structure Formation in Cosmetic and Dermatological Treatments." Advances in Dermatology and Allergology, 2019.
https://www.scienceopen.com/document_file/d7babc4f-6994-46e1-80a7-33c6278cbd82/PubMedCentral/d7babc4f-6994-46e1-80a7-33c6278cbd82.pdf
Nilsson, Charlotte, and Miriam Jacobs.
https://norden.diva-portal.org/smash/get/diva2:1424722/FULLTEXT01.pdf
Retinoid Studies
All material appearing on the ACH GROUP (“content”) is protected by copyright under England & Wales Copyright laws and is the property of the ACH Group or party credited as the provider of the content. You may not copy, reproduce, distribute, publish, display, perform, modify, create derivative works, transmit, or in any way exploit any such content, nor may you distribute any part of this content over any network, including a local area network, sell or offer it for sale, or use such content to construct any kind of database.
You may not alter or remove any copyright or other notice from copies of the content on The ACH Group website. Copying or storing any content except as provided above is expressly prohibited without prior written permission of the ACH Group.
Copyright The ACH Group 2023
